Speak with an attorney experienced in product liability and mass tort cases. They can help you understand your legal options and guide you through the process.
Tell Your State Reps to Vote No on SB30 & HB4806! |
Home - Suboxone Lawsuit
The Suboxone tooth decay multidistrict litigation (MDL) experienced a notable uptick in new filings last month. In January, 142 additional cases were added, bringing the total number of pending lawsuits to 896. However, this figure does not fully capture the extent of the litigation, as many claims remain under the tolling agreement. Despite this, the steady influx of new plaintiffs suggests continued momentum, as reflected in the volume of inquiries received by law firms handling these cases.
In an effort to streamline the progress of the Suboxone MDL, plaintiffs’ attorneys have adjusted their stance on the bellwether trial selection process, making key concessions to align with the defendants’ preferences. They have agreed to a broader initial plaintiff pool, consented to up to 250 depositions, and accepted a provision allowing both parties to strike a trial selection. However, while plaintiffs have compromised, defendants have been slow to uphold their own discovery obligations.
Despite committing to provide search terms and custodial data by January 23, the defendants have yet to fully comply, causing delays in corporate depositions. Meanwhile, plaintiffs have met strict discovery requirements, including the timely submission of medical records, proof of product use, and Census Forms. The defendants’ approach of gradually releasing documents through a vague “rolling production” lacks clarity and raises concerns about prolonged litigation.
Plaintiffs’ attorneys also argue that a bellwether pool of 100 cases is appropriate, in line with previous MDLs. If defendants insist on expanding this number, they should be required to justify the necessity through expert testimony. Given these imbalances in discovery, plaintiffs intend to raise these concerns at upcoming case management conferences to ensure the litigation moves forward efficiently.
During a recent status conference, Judge Calabrese reviewed key developments in the Suboxone litigation, a case involving claims of severe dental issues linked to the opioid addiction treatment drug. These status conferences serve as checkpoints to assess progress and resolve disputes before trial.
A primary topic of discussion was a disagreement over deposition confidentiality. The court provided clear instructions on how attorneys should handle these matters moving forward. Additionally, the judge ensured the accuracy of the latest claimant list.
Attorneys were assigned two critical tasks. First, they must establish a standardized process for collecting medical records, as these are crucial pieces of evidence in the case. Second, they need to finalize the bellwether trial selection process, which will help set the course for future case outcomes. Securing trial dates would mark a major step toward a potential global settlement for Suboxone-related tooth decay claims.
The next status conference is scheduled for February 12, 2025.
Nearly two years into the litigation, the volume of new Suboxone claims remains unexpectedly high. Our legal team continues to sign new plaintiffs at a pace similar to last year, far exceeding early expectations. This ongoing wave of new cases highlights the widespread impact of Suboxone-related dental injuries. Additionally, as more medical records are reviewed, it’s becoming increasingly clear that the extent of harm suffered by many individuals is even more severe than initially anticipated.
An evolving legal argument in the Suboxone litigation takes a different approach to the failure-to-warn issue. Rather than focusing solely on whether patients would have avoided the medication entirely if properly informed, this claim underscores a lost opportunity for harm reduction. Had adequate warnings been provided, patients could have taken preventive measures—such as rinsing their mouths after use, increasing fluoride intake, or attending more frequent dental checkups—to minimize the risk of severe dental damage.
Pharmaceutical manufacturers often defend against failure-to-warn claims by arguing that patients would have taken the medication regardless, making the warning irrelevant. However, in this case, many Suboxone users might have continued their treatment but taken proactive steps to safeguard their dental health had they been properly informed. By failing to disclose these risks, Indivior denied patients the ability to protect themselves. This presents a unique angle in failure-to-warn litigation, differentiating it from more traditional cases.
One key question remains: How does Indivior view the growing wave of litigation? The company has already faced legal scrutiny over Suboxone in the past, including criminal charges, yet continues to profit substantially from the drug.
It’s possible that Indivior believes juries will be less sympathetic toward plaintiffs with addiction histories. However, this assumption may be a critical miscalculation. Juries have historically responded to human suffering, and the powerful personal stories of Suboxone plaintiffs—many of whom followed medical guidance yet suffered severe dental damage—could resonate strongly. If Indivior does not offer fair settlement amounts reflective of these realities, it is likely these cases will proceed to trial rather than being resolved through a global settlement.
A new lawsuit out of East Stroudsburg, Pennsylvania, details the devastating impact of Suboxone film on a plaintiff’s dental health. The individual, prescribed the medication for opioid dependency, developed severe and permanent dental damage, including cavities, cracked teeth, and gum disease.
Despite adhering to the prescribed dosage and maintaining proper oral hygiene, the plaintiff experienced rapid deterioration of their teeth, ultimately requiring fillings, root canals, and extractions. The complaint argues that Suboxone’s formulation—designed to optimize absorption—caused prolonged exposure to acidic conditions, leading to irreversible harm.
In a key ruling for plaintiffs, the MDL judge issued a decision that, while mixed, ultimately benefits those pursuing claims against Indivior. The ruling applied to a single case but is expected to set a precedent for similar claims moving forward.
The judge upheld the plaintiff’s pre-approval design defect claim, affirming that manufacturers had a duty to explore safer alternatives before FDA approval—such as Sublocade, an injectable form of buprenorphine. Additionally, the failure-to-warn claim was allowed to proceed, as Suboxone’s labeling did not warn of dental risks until 2022, leaving patients and healthcare providers unaware of the potential harm.
However, the court dismissed claims related to post-approval design modifications and narrowed the failure-to-warn argument to Indivior, the label-holder. While not a complete victory, this ruling reinforces the manufacturer’s responsibility to ensure its product’s safety and transparency about emerging risks.
For those seeking more information, we continue to provide straightforward, honest answers about Suboxone lawsuits, including insight into potential settlement values and legal options.
A recently filed lawsuit in the Suboxone multidistrict litigation (MDL) features a plaintiff from Madison, Indiana, who was prescribed the medication while in Michigan. The complaint alleges that the drug’s manufacturers, including Indivior and Aquestive Therapeutics, failed to adequately warn users about the severe risk of dental damage associated with Suboxone film.
The lawsuit claims that the defendants were aware of these risks for over a decade but delayed issuing sufficient warnings. The plaintiff points to the 2022 FDA Drug Safety Communication, which confirmed significant dental issues linked to buprenorphine products designed to dissolve in the mouth. Despite this public acknowledgment, the complaint asserts that the manufacturers did not adequately update patient medication guides, leaving users uninformed about potential harm.
Anticipating future legal battles over the statute of limitations, the lawsuit argues that Suboxone still lacks an adequate warning, even after the FDA safety communication. This claim could prove crucial in determining whether plaintiffs who only recently discovered their injuries can still pursue legal action.
Many Suboxone plaintiffs are anxiously awaiting news about potential settlements, but when might payouts actually begin?
While there has been speculation that a settlement could arrive sooner than expected—perhaps even before a Daubert ruling—a more realistic timeline points to mid-2025. This expectation is tied to the legal debate over the three-year statute of limitations for filing claims. Indivior’s legal team argues that this window expires around June 2025, meaning the company may time its settlement strategy accordingly.
Rather than announcing settlements early, Indivior is expected to wait until closer to the statute of limitations cutoff to avoid triggering a surge of new claims. By delaying, the company aims to limit additional liability and better manage the total number of plaintiffs participating in the litigation. As a result, while early settlement discussions may be happening behind the scenes, any speculation about a quick resolution appears premature.
You may be eligible to file a suboxone tooth decay lawsuit if you were:
Our Suboxone attorneys are ready to help you navigate the ins and outs of recovering the compensation you deserve for the damages you suffered as a result of the manufacturer’s negligence.
Suboxone is manufactured by Indivior PLC, a pharmaceutical company specializing in addiction treatment medications. The drug was initially developed by Reckitt Benckiser Pharmaceuticals, which later spun off its pharmaceutical division into Indivior in 2014.
Additionally, Aquestive Therapeutics (formerly known as MonoSol Rx) played a key role in developing the sublingual film formulation of Suboxone, which is at the center of the current litigation over dental injuries.
Indivior remains the primary manufacturer and marketer of Suboxone, while various generic versions of the drug are now available from other pharmaceutical companies.
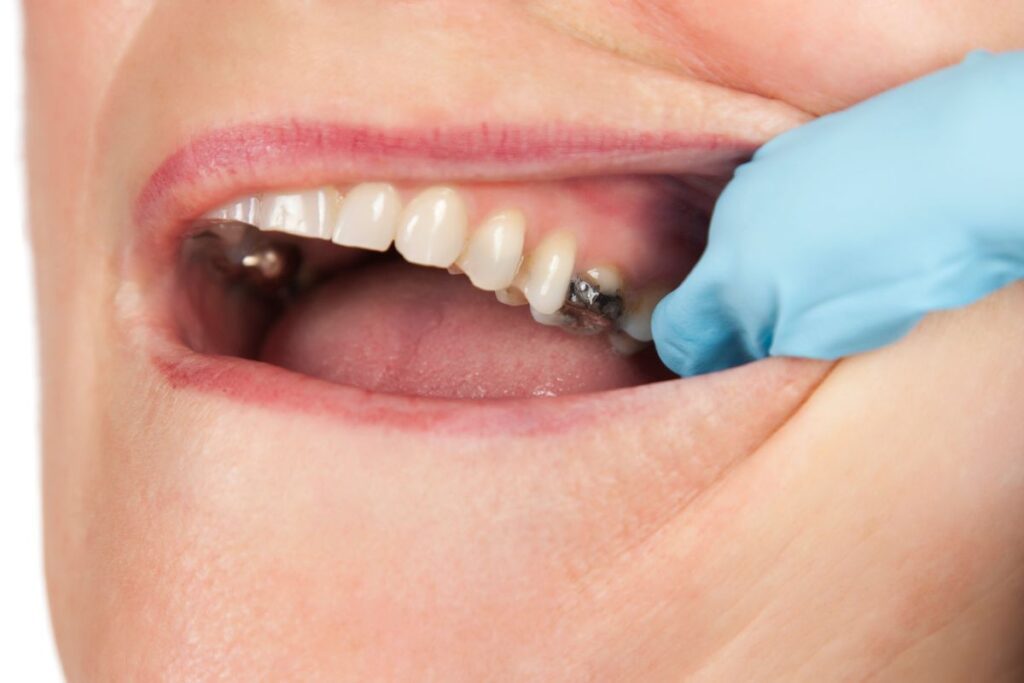
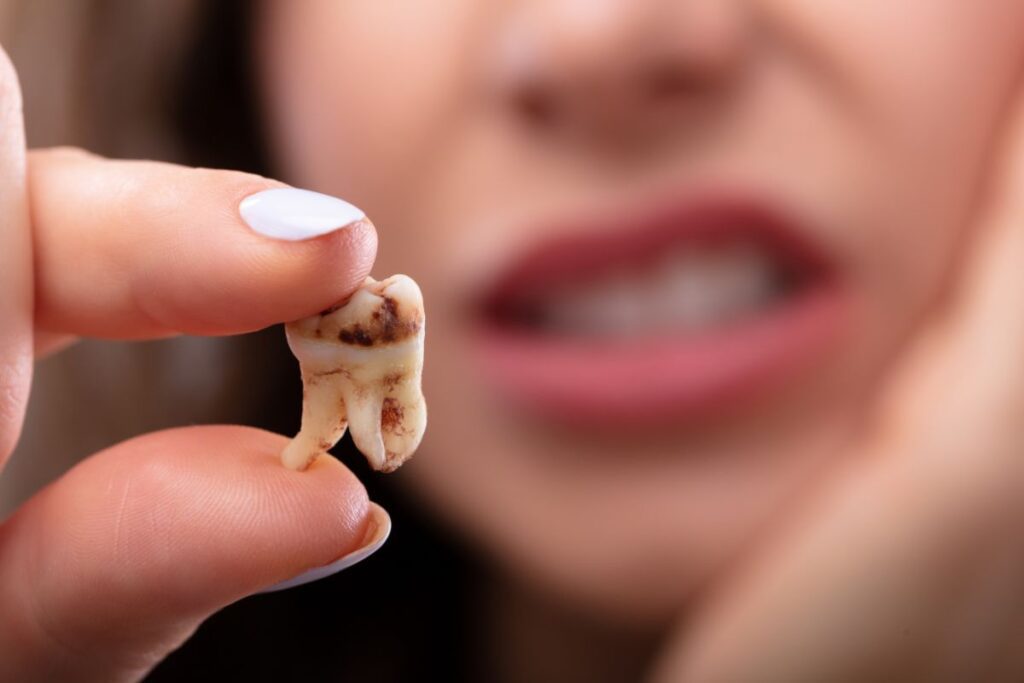
The Suboxone Multidistrict Litigation (MDL) is a consolidation of numerous lawsuits brought by individuals who suffered from dental issues such as tooth loss, dental decay, and other injuries due to the use of Suboxone film. This opioid addiction treatment, when dissolved in the mouth, has been found to have a high acidity level, which can lead to significant tooth decay. The lawsuits claim that the use of Suboxone film has caused severe dental problems, including tooth loss and dental decay, due to its acidic nature.
Understanding the complexities of the Suboxone MDL is crucial for those who may have been affected by its use. If you believe you have experienced dental issues as a result of Suboxone, you may be eligible to participate in this litigation.
Suboxone is a medication widely used to treat opioid addiction.
It is a combination of buprenorphine and naloxone, designed to reduce withdrawal symptoms and cravings in individuals recovering from opioid addiction. The medication is administered as a sublingual film, placed under the tongue to dissolve, making it a convenient and effective treatment option. However, the acidic nature of the sublingual film has been linked to significant dental health issues.
Many users of Suboxone have reported severe dental problems, including:
The Suboxone tooth decay lawsuit is based on claims that Indivior, the manufacturer, failed to provide adequate warnings about the risks of severe dental issues associated with the sublingual film. The FDA mandated these warnings only in June 2022, following numerous patient complaints and reports of dental problems. Plaintiffs argue that earlier warnings could have prevented significant and irreversible dental damage.
If you believe you are eligible for the Suboxone tooth decay lawsuit, follow these steps:
Speak with an attorney experienced in product liability and mass tort cases. They can help you understand your legal options and guide you through the process.
Collect medical and dental records that document your use of Suboxone and the subsequent dental problems you experienced.
Your attorney will help you file a claim and join the MDL if applicable. They will handle the legal proceedings on your behalf, ensuring your rights are protected.
There are statutes of limitations that apply to filing product liability claims. These laws set a time limit on how long you have to file a lawsuit after discovering the injury. Given the recent updates to Suboxone’s warning label, the clock might be ticking for many potential plaintiffs. Consult with an attorney as soon as possible to ensure your claim is filed within the appropriate timeframe.


Plaintiffs in the Suboxone tooth decay lawsuit seek compensation for various damages, including:

We operate on a contingency fee model, ensuring that our clients face no upfront costs or hourly charges. We are solely rewarded based on the success we achieve for you. It’s straightforward: we only receive compensation if your case is won.
The compensation you may receive from a Suboxone lawsuit can vary widely depending on factors like the severity of your dental issues and the details of your case. Estimates suggest that individual settlements may be up into the six figures, depending on the specifics of your case. This figure are speculative and based on previous product liability cases.
Because the lawsuits are still in the early stages, it’s difficult to provide a precise estimate at this time. The final settlement amounts will depend on how the cases proceed through the court system, whether they reach trial, and if any large-scale settlements are negotiated as part of multidistrict litigation.
If you are considering joining a lawsuit, it’s best to consult with an experienced attorney who can assess your specific situation and provide more personalized guidance.
To join the Suboxone lawsuit, follow these steps:
It’s important to act quickly due to potential statute of limitations issues, so consulting with a lawyer as soon as possible is critical to protecting your legal rights.
The timeline for settling the Suboxone lawsuit can vary widely depending on several factors. These include the number of plaintiffs involved, the complexity of the legal issues, and the willingness of the defendant to negotiate a settlement.
In general, if the cases are part of a multidistrict litigation (MDL), like the ongoing Suboxone tooth decay MDL, the process can take several years. This is due to the time needed for gathering evidence, conducting discovery, and preparing for potential trials. Some cases could settle within a year or two if settlements are reached early, while others might take longer, especially if the defendant contests the claims or if trials are necessary.
For individual plaintiffs not part of an MDL, the timeline can also be affected by the specific circumstances of the case, such as the strength of the evidence and the legal strategy of both parties. It’s important to work closely with a lawyer to understand the specifics of your case and get a more accurate estimate.
The Suboxone lawsuit was filed due to claims that the medication, specifically its sublingual film form, causes severe dental problems, such as tooth decay, gum disease, and tooth loss. The plaintiffs allege that the manufacturers, Indivior and its partners, failed to adequately warn users about these potential side effects. Suboxone, which is commonly used to treat opioid addiction, is taken by placing a dissolvable film under the tongue. This method has been linked to creating an acidic environment in the mouth, leading to the erosion of tooth enamel and other dental health issues.
Additionally, there have been accusations against Indivior regarding misleading marketing practices and antitrust violations related to Suboxone, further contributing to the lawsuits. These claims suggest that Indivior promoted the drug without fully disclosing the risks or sought to extend its market exclusivity unfairly.
People affected by these dental issues are seeking compensation for medical costs, pain and suffering, and other damages.
Contact us at 866-779-0356 for a free consultation where our team can answer your questions and help ease your concerns. We have extensive experience dealing with pharmaceutical companies who manufacturer dangerous drugs.

With over a dozen locations throughout Texas, there’s a Carlson Law Firm near you. We have law offices located in Killeen, Temple, Waco, Round Rock, Austin, San Antonio, Kerrville, Laredo, Bryan, Lubbock, Midland, and Corpus Christi.
100 E. Central Texas Expy
Killeen, TX 76541
(254) 526-5688
11606 N. I-35
Austin, TX 78753
(512) 346-5688
135 W. Slaughter Ln, Ste A
Austin, TX 78748
(512) 804-7277
6243 I-10 #550
San Antonio, TX 78201
(210) 696-8600
618 SW Military Dr.
San Antonio, TX 78221
(210) 923-7700
1109 W. Baker Road, Ste A
Baytown, TX 77521
(832) 806-6155
1121 Briarcrest Drive, Ste 200
Bryan, TX 77802
(979) 260-5688
653 Everhart Rd, Ste 105
Corpus Christi, TX 78411
(361) 336-3317
301 Junction Highway, Ste 100
Kerrville, TX 78028
(830) 257-7575
5112 McPherson, Ste 106
Laredo, TX 78041
(956) 712-2588
10101 Quaker Ave
Lubbock, TX 79424
(806) 401-0500
900 Lp 250 Frontage Rd b
Midland, TX 79705
(432) 247-6611
1717 N. I-35 Ste 305
Round Rock, TX 78664
(512) 671-7277
4282 S Jackson St.
San angelo, TX 76903
(325) 238-4322
2010 SW H K Dodgen Loop, Ste 201
Temple TX 76504
(254) 771-5688
2420 I-35 South
Waco, TX 76706
(254) 772-5653
2909 Garnett Ave
Wichita Falls, TX 76308
(940) 285-6333
1500 Rosecrans Ave Ste 500
Manhattan Beach, CA 90266
(254) 516-3570
33rd Ave N Ste 220
St. Petersburg, FL 33701
(727) 373-4655
100 E. Central Texas Expy
Killeen, TX 76541
(254) 526-5688
11606 N. I-35
Austin, TX 78753
(512) 346-5688
135 W. Slaughter Ln, Ste A
Austin, TX 78748
(512) 804-7277
6243 I-10 #550
San Antonio, TX 78201
(210) 696-8600
618 SW Military Dr.
San Antonio, TX 78221
(210) 923-7700
1109 W. Baker Road, Ste A
Baytown, TX 77521
(832) 806-6155
1421 S Main St. Ste 113
Boerne, TX 78006
(830) 470-8777
1121 Briarcrest Drive, Ste 200
Bryan, TX 77802
(979) 260-5688
653 Everhart Rd, Ste 105
Corpus Christi, TX 78411
(361) 336-3317
5112 McPherson, Ste 106
Laredo, TX 78041
(956) 712-2588
10101 Quaker Ave
Lubbock, TX 79424
(806) 401-0500
900 Lp 250 Frontage Rd b
Midland, TX 79705
(432) 247-6611
559 S I-35 Frontage Rd Ste 250,
Round Rock, TX 78664
(512) 671-7277
4282 S Jackson St.
San angelo, TX 76903
(325) 238-4322
2010 SW H K Dodgen Loop, Ste 201
Temple TX 76504
(254) 771-5688
2420 I-35 South
Waco, TX 76706
(254) 772-5653
2909 Garnett Ave
Wichita Falls, TX 76308
(940) 285-6333
1500 Rosecrans Ave Ste 500
Manhattan Beach, CA 90266
(254) 516-3570
4700 Millenia Blvd. STE 500
Orlando, FL 32839
(727) 373-4655