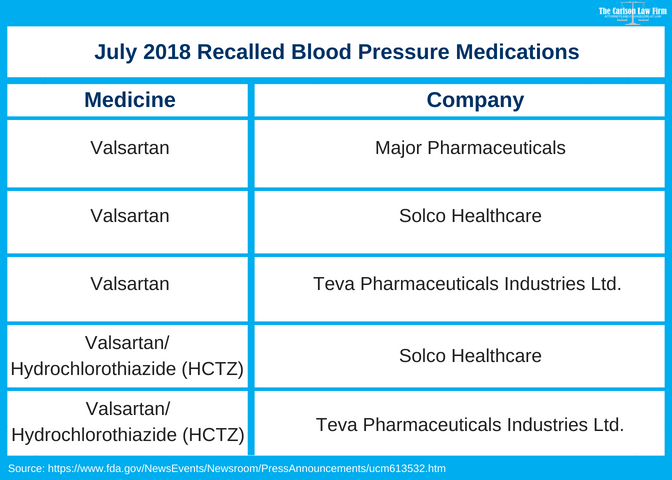
The Food and Drug Administration has announced a voluntary recall of a widely prescribed blood pressure medications over cancer concerns. Major Pharmaceuticals, Solco Healthcare and Teva Pharmaceuticals Industries Ltd. issued a voluntary recall at the FDA’s request. The request came after the Administration found a cancer-causing agent in drugs containing valsartan, a generic drug used to treat high blood pressure.
Three companies are pulling several drug products containing valsartan because of an impurity, N-nitrosodimethylamine (NDMA). NDMA is a probable human carcinogen (a substance that can cause cancer).
The FDA became aware of the issue after lab testing discovered the unexpected presence of NDMA. Experts believe changes in the manufacturing of valsartan—the active ingredient in the medications— is the reason for NDMA’s presence.
What drugs are on the list of recalls?
The FDA is continuing to review and investigate the levels of NDMA found in valsartan drugs. In addition, the agency says it is assessing possible effects on patients who have taken the drugs. Further, it is looking for possible changes to the process that will reduce or eliminate the impurity from future batches.
“The FDA is committed to maintaining our gold standard for safety and efficacy. That includes our efforts to ensure the quality of drugs and the safe manner in which they’re manufactured,” FDA Commissioner Scott Gottlieb, M.D. said. “When we identify lapses in the quality of drugs and problems with their manufacturing that have the potential to create risks to patients, we’re committed to taking swift action to alert the public and help facilitate the removal of the products from the market. As we seek the removal of certain drug products today, our drug shortages team is also working hard to ensure patients’ therapeutic needs are met in the United States with an adequate supply of unaffected medications.”
What should I do with medications containing Valsartan?
The FDA advises several steps to take if you take blood pressure medication:
- View our chart above and determine if your product is one of the recalled medications. You can do so by looking at the drug name and company name on the label of your prescription bottle. If the information is not on the bottle, contact your pharmacy.
- Because valsartan treats serious medical conditions, the FDA advises patients to continue taking the medicines until they have found a replacement medication.
- If a patient taking one of the recalled medications, they should follow the recall instructions provided by the specific company.
- Patients should also contact their doctors or pharmacists to discuss their medication. If your medication is part of the recall, you should ask your doctor or pharmacist if there is an alternative treatment or medication available.
The Carlson Law Firm Can Help
The Carlson Law Firm has more than 40 years representing clients hurt by dangerous drugs. In addition to medical bills, you can expect several other expenses and losses because of dangerous drugs. Call our firm today to schedule a free consultation with a products liability attorney at The Carlson Law Firm.
We are available 24/7.





